Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirano, Tatsumi*; Maeda, Takehiro*; Murata, Tetsuyuki*; Yamaki, Takahiro*; Matsubara, Eiichiro*; Shobu, Takahisa; Shiro, Ayumi*; Yasuda, Ryo*; Takamatsu, Daiko*
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(1), p.49 - 57, 2023/02
 -dispersed LATP by means of neutron radiography
-dispersed LATP by means of neutron radiographySong, F.*; Chen, H.*; Hayashida, Hirotoshi*; Kai, Tetsuya; Shinohara, Takenao; Yabutsuka, Takeshi*; Yao, Takeshi*; Takai, Shigeomi*
Solid State Ionics, 377, p.115873_1 - 115873_6, 2022/04
Times Cited Count:2 Percentile:31.08(Chemistry, Physical)Hamamoto, Shimpei; Ishitsuka, Etsuo; Nakagawa, Shigeaki; Goto, Minoru; Matsuura, Hideaki*; Katayama, Kazunari*; Otsuka, Teppei*; Tobita, Kenji*
Proceedings of 2021 International Congress on Advances in Nuclear Power Plants (ICAPP 2021) (USB Flash Drive), 5 Pages, 2021/10
Impurity concentrations of hydrogen and hydride in the coolant were investigated in detail for the HTTR, a block type high-temperature gas reactor owned by Japan. As a result, it was found that CH was 1/10 of H
was 1/10 of H concentration, which was under the conventional detection limit. If the ratio of H
concentration, which was under the conventional detection limit. If the ratio of H to CH
to CH in the coolant is the same as the ratio of HT to CH
in the coolant is the same as the ratio of HT to CH T, the CH
T, the CH T has a larger dose conversion factor, and this compositional ratio is an important finding for the optimal dose evaluation. Further investigation of the origin of CH
T has a larger dose conversion factor, and this compositional ratio is an important finding for the optimal dose evaluation. Further investigation of the origin of CH suggested that CH
suggested that CH was produced as a result of a thermal equilibrium reaction rather than being released as an impurity from the core.
was produced as a result of a thermal equilibrium reaction rather than being released as an impurity from the core.
Chikhray, Y.*; Askerbekov, S.*; Kenzhin, Y.*; Gordienko, Y.*; Ishitsuka, Etsuo
Fusion Science and Technology, 76(4), p.494 - 502, 2020/05
Times Cited Count:1 Percentile:12.16(Nuclear Science & Technology)Ishitsuka, Etsuo; Kenzhina, I.*; Okumura, Keisuke; Ho, H. Q.; Takemoto, Noriyuki; Chikhray, Y.*
JAEA-Technology 2018-010, 33 Pages, 2018/11
As a part of study on the mechanism of tritium release to the primary coolant in research and testing reactors, tritium recoil release rate from Li and U impurities in the neutron reflector made by beryllium, aluminum and graphite were calculated by PHITS code. On the other hand, the tritium production from Li and U impurities in beryllium neutron reflectors for JMTR and JRR-3M were calculated by MCNP6 and ORIGEN2 code. By using both results, the amount of recoiled tritium from beryllium neutron reflectors were estimated. It is clear that the amount of recoiled tritium from Li and U impurities in beryllium neutron reflectors are negligible, and 2 and 5 orders smaller than that from beryllium itself, respectively.
Goto, Minoru; Okumura, Keisuke; Nakagawa, Shigeaki; Inaba, Yoshitomo; Matsuura, Hideaki*; Nakaya, Hiroyuki*; Katayama, Kazunari*
Fusion Engineering and Design, 136(Part A), p.357 - 361, 2018/11
Times Cited Count:6 Percentile:52.79(Nuclear Science & Technology)A High Temperature Gas-cooled Reactor (HTGR) is proposed as a tritium production device, which has the potential to produce a large amount of tritium using  Li(n,
Li(n, )T reaction. In the HTGR design, generally, boron is loaded into the core as a burnable poison to suppress excess reactivity. In this study, lithium is loaded into the HTGR core instead of boron and is used as a burnable poison aiming to produce thermal energy and tritium simultaneously. The nuclear characteristics and the fuel temperature were calculated to confirm the feasibility of the lithium-loaded HTGR. It was shown that the calculation results satisfied the design requirements and hence the feasibility was confirmed for the lithium-loaded HTGR, which produce thermal energy and tritium.
)T reaction. In the HTGR design, generally, boron is loaded into the core as a burnable poison to suppress excess reactivity. In this study, lithium is loaded into the HTGR core instead of boron and is used as a burnable poison aiming to produce thermal energy and tritium simultaneously. The nuclear characteristics and the fuel temperature were calculated to confirm the feasibility of the lithium-loaded HTGR. It was shown that the calculation results satisfied the design requirements and hence the feasibility was confirmed for the lithium-loaded HTGR, which produce thermal energy and tritium.
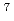 Be(n,p)
Be(n,p)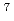 Li reaction and the cosmological lithium problem; Measurement of the cross section in a wide energy range at n_TOF at CERN
Li reaction and the cosmological lithium problem; Measurement of the cross section in a wide energy range at n_TOF at CERNDamone, L.*; Barbagallo, M.*; Mastromarco, M.*; Cosentino, L.*; Harada, Hideo; Kimura, Atsushi; n_TOF Collaboration*; 152 of others*
Physical Review Letters, 121(4), p.042701_1 - 042701_7, 2018/07
Times Cited Count:50 Percentile:91.96(Physics, Multidisciplinary)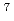 Be(n,p)
Be(n,p)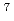 Li reaction at n_TOF
Li reaction at n_TOFBarbagallo, M.*; Andrzejewski, J.*; Mastromarco, M.*; Perkowski, J.*; Damone, L. A.*; Gawlik, A.*; Kimura, Atsushi; n_TOF Collaboration*; 122 of others*
Nuclear Instruments and Methods in Physics Research A, 887, p.27 - 33, 2018/04
Times Cited Count:13 Percentile:79.66(Instruments & Instrumentation)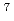 Be(n,
Be(n, ) and
) and 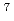 Be(n,p) cross-section measurement for the cosmological lithium problem at the n_TOF facility at CERN
Be(n,p) cross-section measurement for the cosmological lithium problem at the n_TOF facility at CERNBarbagallo, M.*; Colonna, N.*; Aberle, O.*; Harada, Hideo; Kimura, Atsushi; n_TOF Collaboration*; 125 of others*
EPJ Web of Conferences, 146, p.01012_1 - 01012_4, 2017/09
Times Cited Count:1 Percentile:61.21(Nuclear Science & Technology)Kurosaki, Yuzuru*; Yokoyama, Keiichi
Chemical Physics, 493, p.183 - 193, 2017/08
Times Cited Count:5 Percentile:21.9(Chemistry, Physical)Electric field of laser pulses which gives maximum selectivity in the isotope-selective rovibrational excitation of lithium chloride molecules is calculated. Applying the optimal control theory, we calculate optimal electric field to produce mixture of LiCl-35 (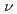 =0,
=0, 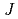 =0) and LiCl-37 (
=0) and LiCl-37 (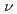 =1,
=1, 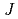 =1) from molecular ensemble of LiCl-35 (
=1) from molecular ensemble of LiCl-35 (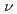 =0,
=0, 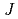 =0) and LiCl-37 (
=0) and LiCl-37 (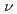 =0,
=0, 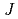 =0). As a result, it is found that electric field which permit rotational excitations only gives high yield in the selective excitation compared to the electric field which permit both rotational and vibrational excitations.
=0). As a result, it is found that electric field which permit rotational excitations only gives high yield in the selective excitation compared to the electric field which permit both rotational and vibrational excitations.
Ichikawa, Shoichi; Chiba, Yusuke; Ono, Fumiyasu; Hatori, Masakazu; Kobayashi, Takanori; Uekura, Ryoichi; Hashiri, Nobuo*; Inuzuka, Taisuke*; Kitano, Hiroshi*; Abe, Hisashi*
JAEA-Research 2017-001, 40 Pages, 2017/03
In order to reduce the influence on a plant schedule of the MONJU by the maintenance of dew point hygrometers, The JAEA examined a capacitance type dew point hygrometer as an alternative dew point hygrometer for a lithium-chloride type dew point hygrometer which had been used at the CV-LRT in the MONJU. As a result of comparing a capacitance type dew point hygrometer with a lithium-chloride type dew point hygrometer at the CV-LRT (Atmosphere: nitrogen, Testing time: 24 hours), there weren't significant difference between a capacitance type dew point hygrometer and a lithium-chloride type dew point hygrometer. As a result of comparing a capacitance dew point hygrometer with a high-mirror-surface type dew point hygrometer for long term verification (Atmosphere: air, Testing time: 24 months), the JAEA confirmed that a capacitance type dew point hygrometer satisfied the instrument specification ( 2.04
2.04 C) required by the JEAC4203-2008.
C) required by the JEAC4203-2008.
Furukawa, Tomohiro; Hirakawa, Yasushi; Kondo, Hiroo; Kanemura, Takuji
Nuclear Materials and Energy (Internet), 9, p.286 - 291, 2016/12
Times Cited Count:5 Percentile:36.53(Nuclear Science & Technology)In order to exchange the components which received irradiation damage during the operation at the International Fusion Materials Irradiation Facility, the adhered lithium, which is partially converted to lithium compounds such as lithium oxide and lithium hydroxide, should be removed from the components. In this study, the dissolution experiments of lithium compounds (lithium nitride, lithium hydroxide, and lithium oxide) were performed in a candidate solvent, allowing the clarification of time and temperature dependence. Based on the results, a cleaning procedure for adhered lithium on the inner surface of the components was proposed.
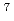 Li(p,xn) reaction for incident energies up to 200 MeV
Li(p,xn) reaction for incident energies up to 200 MeVMatsumoto, Yuiki*; Watanabe, Yukinobu*; Kunieda, Satoshi; Iwamoto, Osamu
JAEA-Conf 2015-003, p.191 - 196, 2016/03
Neutron emission from the 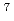 Li(p,n)
Li(p,n)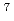 Be reaction can be divided into two components: a mono-energetic component for the transition to the ground and the 1st excited states and a continuum component formed by
Be reaction can be divided into two components: a mono-energetic component for the transition to the ground and the 1st excited states and a continuum component formed by 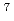 Li breakup processes. For the former, we have obtained the differential cross sections by interpolation based on Legendre fitting of available experimental data up to 45 MeV and apply DWBA calculations above 45 MeV. Next, we have applied the CCONE code to DDX calculations of the continuum component, and adjusted pre-equilibrium model parameters to reproduce experimental data well. Finally, both the results are merged and then the evaluated DDX data are completed.
Li breakup processes. For the former, we have obtained the differential cross sections by interpolation based on Legendre fitting of available experimental data up to 45 MeV and apply DWBA calculations above 45 MeV. Next, we have applied the CCONE code to DDX calculations of the continuum component, and adjusted pre-equilibrium model parameters to reproduce experimental data well. Finally, both the results are merged and then the evaluated DDX data are completed.
 P
P S
S metastable crystal
metastable crystalMori, Kazuhiro*; Enjuji, Keigo*; Murata, Shun*; Shibata, Kaoru; Kawakita, Yukinobu; Yonemura, Masao*; Onodera, Yohei*; Fukunaga, Toshiharu*
Physical Review Applied (Internet), 4(5), p.054008_1 - 054008_6, 2015/11
Times Cited Count:40 Percentile:82.07(Physics, Applied)no abstracts in English
Furukawa, Tomohiro; Hirakawa, Yasushi; Kondo, Hiroo; Kanemura, Takuji; Wakai, Eiichi
Fusion Engineering and Design, 98-99, p.2138 - 2141, 2015/10
Times Cited Count:6 Percentile:45.92(Nuclear Science & Technology)In the International Fusion Materials Irradiation Facility (IFMIF), a back plate of the target assembly will be exchanged during the in-service period. During the works, the lithium components will react chemically with the surrounding atmosphere. In this research, the chemical reaction of lithium in air, oxygen and nitrogen containing variable humidity at room temperature has been investigated to estimate the chemical reaction during the exchange works.
Katayama, Kazunari*; Ushida, Hiroki*; Matsuura, Hideaki*; Fukada, Satoshi*; Goto, Minoru; Nakagawa, Shigeaki
Fusion Science and Technology, 68(3), p.662 - 668, 2015/10
Times Cited Count:16 Percentile:79.46(Nuclear Science & Technology)Tritium production utilizing nuclear reactions by neutron and lithium in a high-temperature gas-cooled reactor is attractive for development of a fusion reactor. From viewpoints of tritium safety and production efficiency, tritium confinement technique is an important issue. It is known that alumina has high resistance for gas permeation. In this study, hydrogen permeation experiments in commercial alumina tubes were conducted and hydrogen permeability, diffusivity and solubility was evaluated. By using obtained data, tritium permeation behavior from an Al O
O -coated Li-compound particle was simulated. Additionally, by using literature data for hydrogen behavior in zirconium, an effect of Zr incorporation into an Al
-coated Li-compound particle was simulated. Additionally, by using literature data for hydrogen behavior in zirconium, an effect of Zr incorporation into an Al O
O coating on tritium permeation was discussed. It was indicated that the majority of produced tritium was released through the Al
coating on tritium permeation was discussed. It was indicated that the majority of produced tritium was released through the Al O
O coating above 500
coating above 500 C. However, it is expected that total tritium leak is suppressed to below 0.67% of total tritium produced at 500
C. However, it is expected that total tritium leak is suppressed to below 0.67% of total tritium produced at 500 C by incorporating Zr fine particles into the inside of Al
C by incorporating Zr fine particles into the inside of Al O
O coating.
coating.
Kondo, Hiroo; Kanemura, Takuji; Furukawa, Tomohiro; Hirakawa, Yasushi; Wakai, Eiichi
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 8 Pages, 2015/05
A liquid-Li free-surface stream is to serve as a beam target (Li target) for the IFMIF. As a major activity for the Li target in the IFMIF/EVEDA, the EVEDA Li test loop (ELTL) was constructed. This study focuses on cavitation-like acoustic noise in a conduit downstream of the Li target. This noise was detected by using acoustic-emission sensors. The intensity of the noise was examined versus cavitation number of the Li target. In addition, a time-frequency analysis for the acoustic signal was performed to characterize the noise. The results are as follows: (1) the intensity of the noise was increased as decreasing the cavitation number; (2) the noise was at first intermittent in a larger cavitation number, subsequently the noise became continuous as decreasing the cavitation number; (3) the noise consisted of a number of a high frequency acoustic emission which occurred in a short duration. For these results, we conclude that cavitation occurred in the downstream conduit.
Wakai, Eiichi; Ando, Masami; Okubo, Nariaki
Journal of Plasma and Fusion Research SERIES, Vol.11, p.104 - 112, 2015/03
The reduced-activation ferritic/martensitic (RAFM) steels for the fusion DEMO reactor have been developing from around the 1980s. RAFM steels are the first candidate materials for the first wall and blanket structure of fusion DEMO reactors, the target back-plate and the target assembly of IFMIF. In this study, two subjects had been examined and are summarized as below: (1) Effect of initial heat treatment on the microstructures and mechanical properties of RAFM steels, including irradiation damage, is very important to design the fusion DEMO reactors and also control the changes of mechanical properties after the irradiation. (2) Effects of He and H production on the microstructures and mechanical properties of RAFM steels, including irradiation damage, are essential in the evaluation of design of fusion DEMO reactor, and we have to check and evaluate them in Fusion irradiation environment like IFMIF.
 -LiAl; Relation to the defect structure
-LiAl; Relation to the defect structureSugai, Hiroyuki
Solid State Ionics, 177(39-40), p.3507 - 3512, 2007/01
The diffusion coefficient and its activation energy (116.3  11.7 kJ/mol) of tritium in an intermetallic compound
11.7 kJ/mol) of tritium in an intermetallic compound  -LiAl are determined at temperatures from 700 to 848 K. Though the present result for the diffusion coefficient is almost the same as that reported previously, the present result for the activation energy turns out nearly twice of that (64.9
-LiAl are determined at temperatures from 700 to 848 K. Though the present result for the diffusion coefficient is almost the same as that reported previously, the present result for the activation energy turns out nearly twice of that (64.9  3.8 kJ/mol). The present result for the activation energy is consistent with the systematics that an increase of lithium concentration in Al-Li systems increases the activation energy, but the previous result is not. Furthermore, a consideration of the crystal structure and defect structure suggests that tritium diffuses and is impeded by the attractive interaction with lithium atom at lithium sublattices.
3.8 kJ/mol). The present result for the activation energy is consistent with the systematics that an increase of lithium concentration in Al-Li systems increases the activation energy, but the previous result is not. Furthermore, a consideration of the crystal structure and defect structure suggests that tritium diffuses and is impeded by the attractive interaction with lithium atom at lithium sublattices.
 -LiAl; Relation to the defect structure
-LiAl; Relation to the defect structureSugai, Hiroyuki
Solid State Ionics, 177(39-40), p.3507 - 3512, 2007/01
Times Cited Count:3 Percentile:17.87(Chemistry, Physical)The diffusion coefficients and its activation energy (103.7 9.5 kJ/mol) for tritium in intermetallic compound
9.5 kJ/mol) for tritium in intermetallic compound  -LiAl are determined at temperatures from 699 to 886 K. Though the present result for the diffusion coefficient is almost the same as that reported earlier, the activation energy turns out nearly twice of that (64.9
-LiAl are determined at temperatures from 699 to 886 K. Though the present result for the diffusion coefficient is almost the same as that reported earlier, the activation energy turns out nearly twice of that (64.9 3.8 kJ/mol) reported earlier. On the basis of the crystal structure and defect structure, the large activation energy of this study suggest that tritium diffuses interstitially and is impeded by an attractive interaction with lithium atoms in lithium sublattices.
3.8 kJ/mol) reported earlier. On the basis of the crystal structure and defect structure, the large activation energy of this study suggest that tritium diffuses interstitially and is impeded by an attractive interaction with lithium atoms in lithium sublattices.